Original source: Science
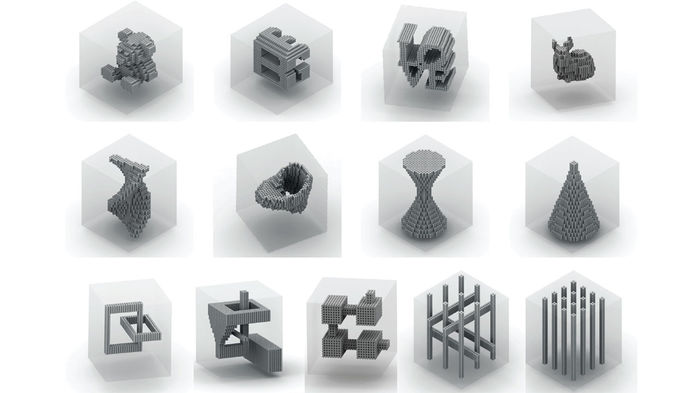
Scientists have made a big advance in building shapes out of the so-called building blocks of life. New techniques can shape DNA—the double-stranded helical molecule that encodes genes—into objects up to 20 times bigger than previously achieved, three separate groups report today. Together, the new approaches can make objects of virtually any shape: 3D doughnuts and dodecahedrons, cubes with teddy bear–shaped cutouts, and even a tiled image of the Mona Lisa. The techniques could someday lead to a bevy of novel devices for electronics, photonics, nanoscale machines, and possibly disease detection.
Scientists have been making shapes out of DNA since the 1980s, and those efforts took off in 2006 with the invention of a folding technique called DNA origami. It starts with a long DNA strand—called a scaffold—that has a precise sequence of the four molecular units, or nucleotides, dubbed A, C, G, and T, with which DNA spells out its genetic code. Researchers match patches of the scaffold to complementary strands of DNA called staples, which latch on to their targets in two separate places. Connecting those patches forces the scaffold to fold into a prescribed shape. A second version of the technology, introduced in 2012, uses only small strands of DNA—but no scaffolds—that assemble into Lego-like bricks that can then be linked together.
Both approaches have been wildly popular among nanotechnologists, allowing them to design shapes made from DNA from the bottom up. Researchers have also been able to coat their DNA objects with plastics, metals, and other materials to fashion tiny machine components, electronics, and photonic devices. But the size of conventional DNA objects has been limited to about 100 nanometers: Grow them any larger, and they become too floppy to take a particular shape or cannot make enough connections to their neighbors to get bigger.
Not anymore. Groups in Germany, Massachusetts, and California all report today in Nature that they’ve made DNA objects with newfound heft. The German team, led by Hendrik Dietz, a biophysicist at the Technical University of Munich, modified the traditional origami approach, using it to create rigid DNA modules with preprogrammed shapes that can assemble with other copies to build specific shapes. For example, in solution, DNA strands designed to fold up into 3D wedges combine with one another to form a miniature doughnut about 300 nanometers across. And modules that form three-pronged vertices assemble into various objects, including dodecahedrons that are 440 nanometers wide.
The Massachusetts team, led by Peng Yin, a systems biologist at Harvard University’s Wyss Institute in Boston, modified the DNA brick approach, which they invented, to make larger, more complex structures. In the original technique, each Lego-like brick has a DNA “linker” eight nucleotides long that locks it in place with its neighbors. The new approach uses links of 13 nucleotides each. In their paper, Yin and his colleagues explain how to create a block composed of 33,000 bricks and 1.7 million DNA nucleotides. By leaving out bricks in the center of such blocks, they also created cutouts shaped like everything from an hourglass to a teddy bear. Though such void structures aren’t yet useful, the technique “gives us the ability to engineer highly complex systems,” Yin says.
The third group, led by Lulu Qian, a biochemist at the California Institute of Technology in Pasadena, came up with a new way to create flat DNA origami–based images. Using a multistage assembly process, the researchers created origami-based pixels that appear in different shades when viewed with a device called an atomic force microscope. They assembled dozens of pixels into individual arrays. They then tiled together 64 separate arrays to render an image of the Mona Lisa composed of more than 8700 pixels measuring 0.5 micrometers on a side.
Researchers have previously shown that they can decorate smaller DNA creations with everything from nanoscale metal particles to fluorescent chemical compounds, which may one day be useful for making novel electronic and photonic devices. Because the bigger DNA origami gives researchers more room to sculpt, it opens the door to making ever-more-complex templates for the coatings, Yin says. “We’re collectively making a leap in terms of scale and usefulness of these systems,” he says.
It’s only a matter of time before the objects get even bigger. Yin’s group stopped growing its structures not so much because of technical limitations, but because of the high cost of synthesizing all the DNA—which can carry a price tag of more than $100,000 per gram. Yet another technique from Dietz and his colleagues, also published today in Nature, could lower that cost barrier. The researchers created thousands of tailored DNA strands at once by coaxing viruses to replicate the strands inside bacterial hosts, they report. Similar to how biotech experts generate large quantities of genetically engineered proteins for drugs, this batch method could slash the cost of DNA synthesis to roughly $200 per gram, Dietz says.
Taken together, the new approaches “unlock the application potential of DNA origami,” says Carlos Castro, a DNA nanotech expert at The Ohio State University in Columbus. Other researchers have already loaded hollow origami structures with drugs to target specific types of cancer cells, created DNA “robots” that walk across surfaces, and mimicked the shape of viruses. But because the new DNA objects are on the same size scale of devices that can be patterned using computer chip lithography, it might be possible to integrate the two technologies and design DNA origami to detect cancer biomarkers and other biological targets that could then be read out by electronic devices, Castro says. And that’s just one possible set of devices. Adds Yin: “Now, there are so many ways to be creative with these tools.”