Original source: Materials Today
A printable bioceramic incorporating a mixture of lithium, calcium, and silicon ions supports the regeneration of cartilage and bone tissue simultaneously, according to researchers [Chen et al., Biomaterials (2018), https://doi.org/10.1016/j.biomaterials.2018.04.005].
Degenerative diseases and damage affecting cartilage can extend into the underlying bone, which is known as subchondral tissue, particularly around the joints. As cartilage has poor self-healing capabilities, this type of articular cartilage damage is particularly challenging for orthopedic and sports medicine.
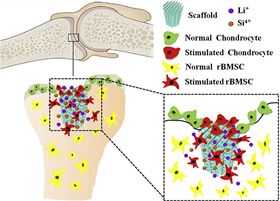
Since cartilage and subchondral bone tissue have different components and biological properties, it has been difficult to design and fabricate a scaffold material that can support the regrowth of both types of tissue at the same time. Now, however, Chengtie Wu and his colleagues from Shanghai Institute of Ceramics, Chinese Academy of Sciences, and Nanjing Medical University Nanjing Hospital have developed a pure-phase lithium calcium silicate (Li2Ca4Si4O13 or L2C4S4 for short) that could be a promising biomaterial for reconstructing defects and damage at the cartilage-osteochondral interface.
The researchers incorporated a number of known bioactive components into the new material. Si plays an important role in the initial mineralization phase of young bone formation and silicate biomaterials stimulate the proliferation and bone-related gene expression of stem cells, which could enhance osteogenesis and bone tissue regeneration. The salt LiCl stimulates subchondral bone formation and appears to protect cartilage from degradation. Recent research has hinted that Li could also stimulate the proliferation of chondrocytes.
“The combination of several important nutrient elements may have important effects for the two tissues,” explains Wu.
The team devised a simple wet chemical sol-gel method to produce high quality powders of the bioceramic L2C4S4, which are then incorporated into inks for 3D printing. This enables a high degree of control over the porosity and pore size of the scaffold, which the researchers varied from 170 microns to 400 microns. Not only are the resulting scaffolds 2-3 times stronger than other 3D-printed bioceramic scaffolds, their mechanical properties are comparable with natural bone.
“The L2C4S4 scaffolds significantly accelerated cartilage regeneration as well as promoted subchondral bone reconstruction,” points out Wu.
The scaffolds are strong and stable enough to provide a good support for initial bone tissue formation, while the pores provide both an easy path for cell migration or transport and plenty of space for bone ingrowth. The L2C4S4 scaffolds show significant apatite mineralization and growth of subchondral bone in vivo, but are biodegradable once they have done their job.
The dual-activity bioceramic L2C4S4 scaffold offers a smart solution to the regeneration of osteochonral defects, believes Wu.